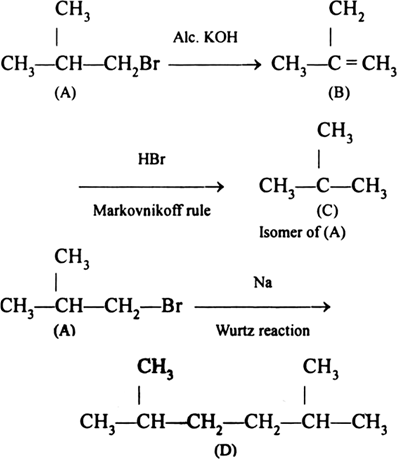
Primary alkyl halide C4H9Br (a) reacted with alcoholic KOH to give compound (b). Compound (b) is treated with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium metal it gives a compound (d), C8H18 that was different from the compound when n-butyl bromide was reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
There can be only two primary alkyl bromides with molecular formula C4H9Br.

According to the problem when alkyl bromide (a) was treated with sodium, it gave a compound which is not a straight chain hydrocarbon. Therefore (a) cannot be butyl bromide. It may be isobutyl bromide. The complete reactions are
1219 Views


