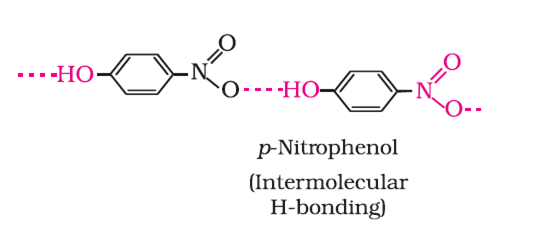
p-nitrophenol is more soluble than ortho and para nitrophenol because P-nitrophenol has Inter-molecular Hydrogen Bonding while o-nitrophenol has Intra-molecular Hydrogen Bonding.
Now due to the Hydrogen Bonding more energy needs to be supplied to vaporize the compound as there are extra bonds other than that present bonds that hold the molecule.
There are bonds between molecules in p-nitrophenol which leads to higher boiling point as more energy need to be supplied to separate the molecules. Whereas in o-nitrophenol the bonds exist in the molecule itself due to which there is virtually no change in the boiling point.