Which is a stronger acid and why? Nitroacetic acid or chloroacetic acid?
Electron withdrawing group increase the acidity of carboxylic acid by stabilising conjugate base through delocalisation of the negative charge. Both nitro and chloro group are electron withdrawing group. O2NCH2COOH is a stronger acid than ClCH2COOH because NO2 is a stronger electron withdrawing group than Cl due to +ve charge on N.
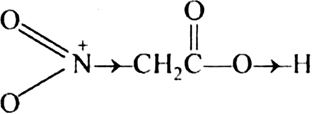
216 Views
