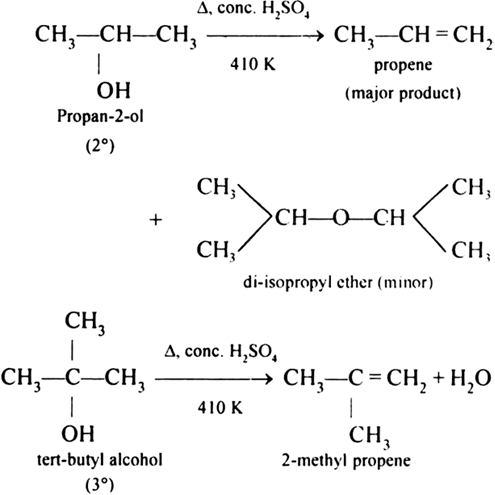
The formation of ethers by dehydration of alcohol is a bimolecular reaction (SN2) involving the attack of alcohol molecule on a protonated alcohol molecule. In the method, the alkyl group should be unhindered. In case of secondary or tertiary alcohols, the alkyl group is hindered. As a result, elimination dominates substitution. Hence, in place of ethers, alkenes are formed.