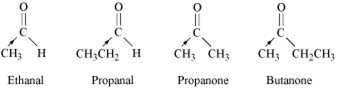
The electron density at the carbonyl carbon increase with the increase in the +I effect. The + I effect of the alkyl group increases in the order:
Ethanal < propanal < propanone < Butanone
As a result, the chances of attack by a nucleophilie decrease. Hence, the increasing order of the reactivates of the given carbonyl compounds in nucleophilic addition reaction is:
Butanone < Propanone < Propanal < Ethanal.