Give plausible explanation for each of the following:
Cyclohexanone forms cyanohydrin in good yield but 2, 2, 6-trimethyl cyclohexanone does not.
The carbonyl group in cyclohexanone is highly polarised and nucleophilic addition of Hδ+—CNδ- at carbonyl group (> Cδ+ = Oδ-) takes place easily.
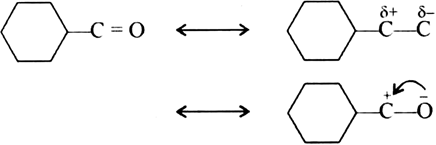
Centre of nucleophile attack by CN- .The presence of three methyl group (which are electron repelling) reduces the polarity of > C = O group on one hand and after a steric hindrance to nucleophilic attack of CN-at > C = O group. Therefore, trimethyl cyclohexanone does not give good yield i.e., it gives very poor yield.
202 Views

