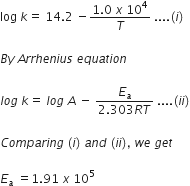The rate constant for the first-order decomposition of H2O2 is given by the following equation: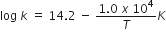
Calculate Ea for this reaction and rate constant k if its half-life period be 200 minutes. (Given: R = 8.314 JK–1mol–1).
Given:
Order of the reaction = First order
t1/2 = 200 minutes = 200 × 60 = 12,000 seconds The relation between t1/2
and k is given by t1/2 = 0.693/k
k = 0.693/12000 = 5.7 × 10−5
The rate constant for the first-order decomposition of H2O2 is given by