(a) For the complex [Fe(CN)6]3–, write the hybridization type, magnetic character and spin nature of the complex. (At. number : Fe = 26).
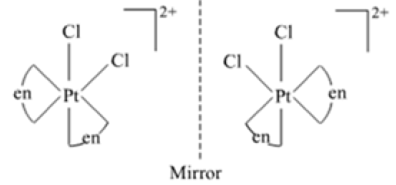
(b) Draw one of the geometrical isomers of the complex [Pt(en)2Cl2]2+ which is optically active.
The electronic configuration of Fe is Ar[18] 4s2 3d6
The electronic configuration of Fe3+ is Ar[18]3d5 4s0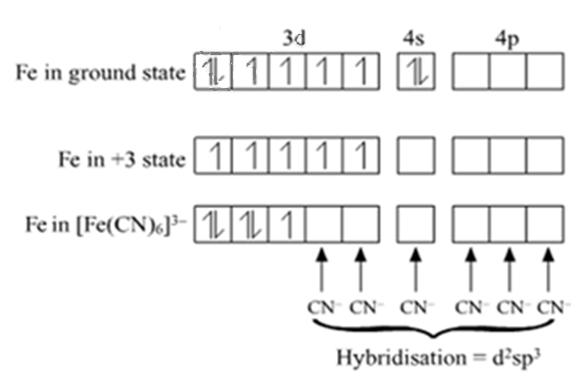
Hybridisation: d2sp3
Magnetic character: Paramagnetic
Spin nature of complex: Low-spin complex
(b) cis-isomer of [Pt(en)2Cl2]2+ is optically active.