(i) Write the IUPAC name of the complex [Cr(NH3)4Cl2]Cl.
(ii) What type of isomerism is exhibited by the complex [Co(en)3]3+?
(en = ethane-1,2-diamine)
(iii) Why is [NiCl4]2− paramagnetic but [Ni(CO)4] is diamagnetic?
(At. nos. : Cr = 24, Co = 27, Ni = 28)
(i) The IUPAC name of the complex [Cr(NH3)4Cl2]Cl is Tetraamminedichlorochromium(III) chloride.
(ii) The complex [Co(en)3]3+ exhibits optical isomerism. Its optical isomers are shown below.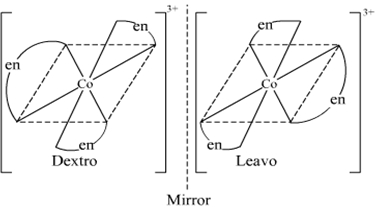
(iii) In [NiCl4]2−, the oxidation state of Ni is +2. Chloride is a weak field ligand and does not cause pairing up of electrons against the Hund's rule of maximum multiplicity. As a result, two unpaired electrons are present in the valence d-orbitals of Ni which impart paramagnetic character to the complex. On the other hand, carbonyl is a strong field ligand and causes pairing up of electrons against the Hund's rule of maximum multiplicity. As a result, no unpaired electrons are present and hence, the complex is diamagnetic.
