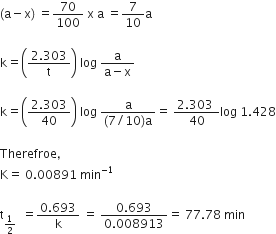A first order reaction takes 40 minutes for 30% decomposition. Calculate t1/2 for this reaction.
(Given log 1.428 = 0.1548)
A --> P
T=0 a 0
T=t (a-x) x
Now, it takes 40 min for 30% decomposition i.e. reactant left after 40 min is 70% of its initial concentration.
So,