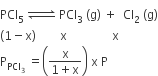Phosphorus pentachloride dissociates as follows, in a closed reaction vessel,
PCl5 (g) ⇌ PCl3(g) + cl2(g)
If total pressure at equilibrium of the reaction mixture is P and degree of dissociation of PCl5 is x, the partial pressure of PCl3 will be
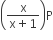
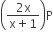
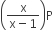

A.