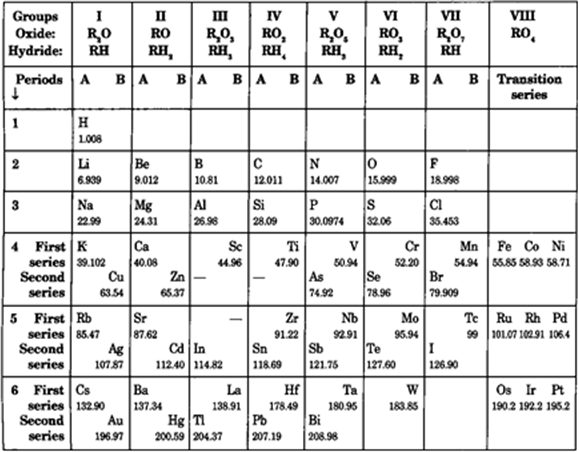
Mendeleev arranged the elements in horizontal rows (known as periods) in the increasing order of their atomic masses. Each vertical column (called groups) contained elements of similar properties. The main features of Mendeleev's periodic table are:
Groups and sub-groups: There are eight groups (I to VIII). Zero group was not present. With the exception of group VIII, each of the remaining groups are sub-divided into sub-groups A and B.
Periods: Horizontal rows are called periods. There are seven periods numbered as 1, 2, 3, 4, 5, 6 and 7. The number of elements in each period are:
1st period—2 elements
2nd period and 3rd period—8 elements each (short period)
4th period and 5th period—18 elements each (long period)
6th period—32 elements including 14 rare earth elements.
7th period—Incomplete period.
Table 5.1: Based on Mendeleev arrangement of elements in 1871