The following table shows the position of six elements A, B, C, D, E and
F in the periodic table.
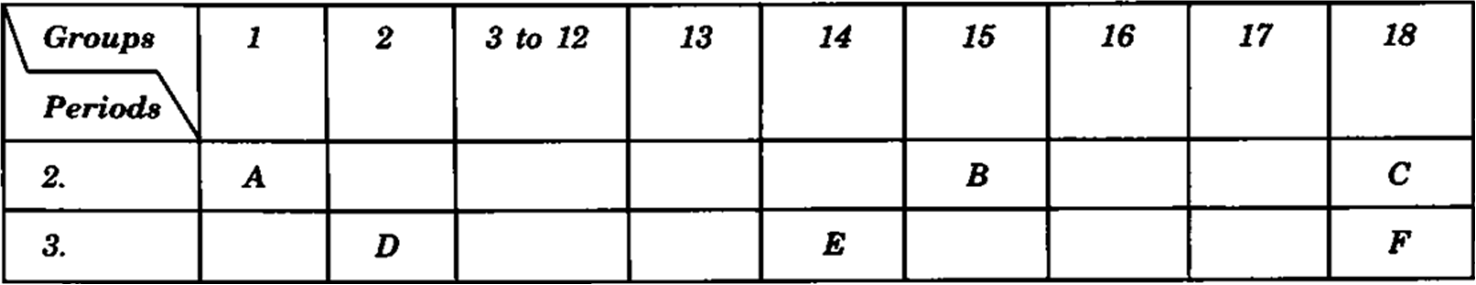
Using the above table answer the following questions:
(a) Which element will form only covalent compounds?
(b) Which element is a metal with valency 2?
(c) Which element is a non-metal with valency of 3?
(d) Out of D and E, which one has a bigger atomic radius and why?
(e) Write a common name for the family of elements C and F.
(a) Element, E will form only covalent compounds.
(b) Element D have 2 valency.
(c) B is non-metal, having 3 valency.
(d) D has bigger atomic radius than E. Both belong to third period. In a period, the atomic radius decreases from left to right
e) C and F belong to noble gas family.
