A student strongly heated green ferrous sulphate (FeSO4.7H2O) solid in a dry test tube. He found that after sometime reddish brown residue was left and some pungent smelling gas evolved. Explain the chemical reaction and write balanced chemical equation.
On heating ferrous sulphate, SO2 and SO3 are produced leaving behind reddish brown ferric oxide.
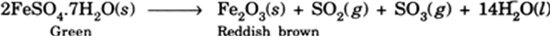
2124 Views
