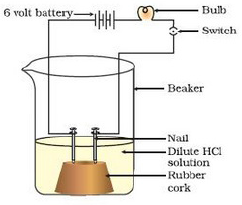
Compounds such as alcohols and glucose also contain hydrogen but are not categorised as acids. Describe an activity to prove it.
Take solutions of alcohols, glucose in a beaker. Take a cork and fix two nails on the cork upto the end. Keep this cork in the beaker. Connect the nails to two terminals of a 6 volt battery through a bulb and a switch. Switch on the current. You will see that bulb does not glow or the current does not pass through the circuit. This means no ions or H+ ions are present in the solution. This shows that alochols and glucose are not acids.
363 Views

