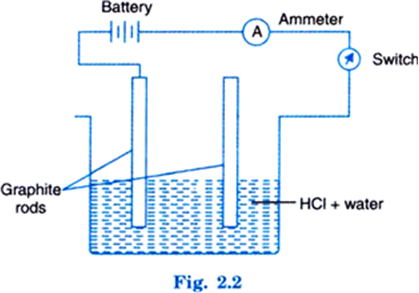
Now switch on the circuit. It will be seen that the pointer of ammeter moves. This shows that current is passing through circuit. This is possible when ions are present in solution.
Now take dry HCl in acetone or any other organic liquid. Arrange the system as before. It will be seen that pointer of the ammeter does not move when circuit is completed. This means no current is passing and solution does not contain ions. Hence, it is inferred that acids produce ions in water solution only.
