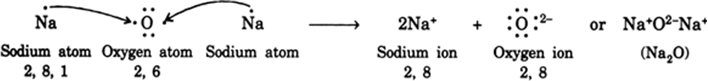(a) Show the formation of Na2O by the transfer of electrons between the combining atoms.
(b) Why are ionic compounds usually hard?
(c) why ionic compounds in the solid state do not conduct electricity and they do so when in molten state?
(a) A sodium atom (2, 8, 1) loses one electron to form a sodium cation. But an oxygen atom (2, 6) can become an oxide ion O2– only when it accepts 2 electrons. Thus it requires two sodium atoms to lose one electron each. In this case the doubly negative charge of oxide anion is balanced exactly by two single positively charged sodium cations. Therefore, there are two cations (Na+) to each oxygen anion (O2–) and the ratio of sodium ions to oxide ions is 2: 1. This is indicated as given below:
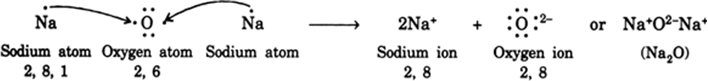
(b) These are relatively hard because these are present as aggregates of several positive and negative ions combined together by strong electrostatic forces.
(c) In solid state, ions in ionic compounds are not mobile while these become mobile in molten state.
1753 Views