Soaps or detergents consist of two parts with distinct properties. One part or end consists of a large hydrocarbon tail which is hydrophobic (water repelling). The other part or end is negatively charged which is hydrophobic (water loving). When a soap is added to water, it does not form a true solution but is dispersed in a way that the ionic end is in water and the hydrophobic part protruding out of water. Inside water, this gives rise to formation of clusters of molecules in which the hydrophobic tails are in the interior of the cluster and the ionic ends are on the surface of the cluster. This formation with a hundreds of soap molecules is called a micelle.
Soap in the form of a micelle is able to clean dirts or oily greese, since it will be collected in the centre of the micelle. When water is agitated, the micelle with the oily dirt tend to lift off from the dirty surface and splits into fragments. This gives opportunity to other tails to stick to oil, if left. The negatively charged heads present in water prevent the small globules so formed from forming aggregates/precipitate because of ion-ion repulsion. Thus the dirt suspended in micelles is easily rinsed away.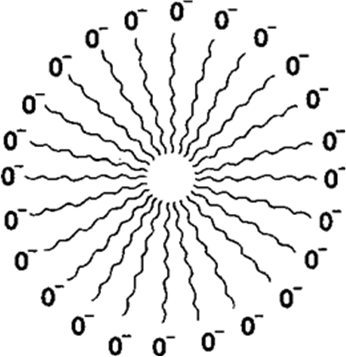
Fig. 4.5. Soap Micelle. Non-polar hydrocarbon chains “dissolve” in each other. Polar —COO— groups dissolve in water. Similarly charged micelles repel each other.
