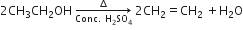(a) In a tabular form, differentiate between ethanol and ethanoic acid under the following heads:
(i) Physical state
(ii) Taste
(iii) NaHCO3 test
(iv) Ester test
(b) Write a chemical reaction to show the dehydration of ethanol.
(a)
|
Property |
Ethanol |
Ethanoic acid |
|
|
(i) |
Physical state |
Ethanol is a colorless liquid with pleasant odor |
Ethanoic acid is colorless, pungent smelling liquid |
|
(ii) |
Taste |
Ethanol is bitter to taste |
Ethanoic acid is sour to taste |
|
(iii) |
NaHCO3 test |
Ethanol does not react with sodium bicarbonate |
When ethanoic acid reacts with sodium NaHCO3 with the evolution of carbon dioxide gas. |
|
(iv) |
Ester test |
Ethanol on reaction with ethanoic acid in the presence of acid forms ester. |
Ethanoic acid on reaction with alcohols in the presence of conc. Sulphuric acid to form ester./ |
(b) Ethanol undergoes dehydration to form ethane.