A student takes 2 mL acetic acid in a dry test tube and adds a pinch of sodium hydrogen carbonate to it. He makes the following observations:
I. A colorless and odorless gas evolves with a brisk effervescence.
II. The gas turns lime water milky when passed through it.
III. The gas burns with an explosion when a burning splinter is brought near it.
IV. The gas extinguishes the burning splinter that is brought near it.
The correct observations are:
I, II, and III
II, III and IV
III, IV and I
III, IV and I
D.
III, IV and I
Reason: When 2 mL acetic acid is taken in a dry test tube and a pinch of sodium hydrogen carbonate is added to it, a colorless and odorless gas evolves with a brisk effervescence which is CO2. 
When CO2 is passed through lime water it turns lime water turns milky.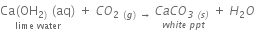
CO2 also extinguishes the burning splinter when it is brought near it.
