Define ionisation energy.
How would the ionisation energy change when electron in hydrogen atom is replaced by a particle of mass 200 times that of the electron but having the same charge ?
The minimum energy, required to free the electron from the ground state of the hydrogen atom, is known as ionization energy.
Ionisation energy is given by,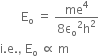
Since, ionisation energy is directly proportional to mass, the Ionization energy will become 200 times.
