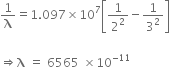A 12.5 eV electron beam is used to bombard gaseous hydrogen at room temperature. Upto which energy levels the hydrogen atoms would be excited?
Calculate the wavelengths of the first member of Lyman and first member of Balmer series.Amount of energy required to excite the electron = 12.5 eV
Energy of the electron in the nth state of an atom = 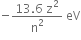 ; Z is the atomic number of the atom. [Z=1 for hydrogen atom]
; Z is the atomic number of the atom. [Z=1 for hydrogen atom]
Energy required to excite an atom from the initial state (ni) to the final state (nf) = 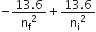
This energy must be equal to or less than the energy of the incident electron beam.
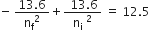 … (1)
… (1)
i=1 for ground state of hydrogen atom.
Energy of the electron in the ground state = −13.6 eV
Now, putting this in equation (1),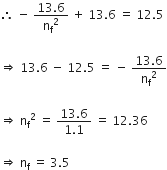
Since, the state cannot be a fractional number we have nf = 3
Therefore, hydrogen atom would be excited up to 3rd energy level.
b) Rydberg formula is given by, 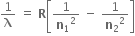 ;
;  is the wavelength and R is the Rydberg constant.
is the wavelength and R is the Rydberg constant.
R = 1.097373157 × 10 7 m-1
For the first member of Lyman series, i=1; f=2
So, 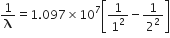
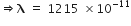
For the first member of the Balmer series, i=2; f=3
So,