a) Deduce the expression, N = N0 e−λt, for the law of radioactive decay.
(b) (i) Write symbolically the process expressing the β+ decay of  . Also write the basic nuclear process underlying this decay.
. Also write the basic nuclear process underlying this decay.
 , an isotope or isobar?
, an isotope or isobar?
As per the law of radioactive decay, we have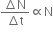
where,
N = Number of nuclei in the sample
ΔN = Amount of nuclei undergoing decay
Δt = Time taken for decay
So,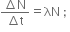 where λ is Decay constant or disintegration constant.
where λ is Decay constant or disintegration constant.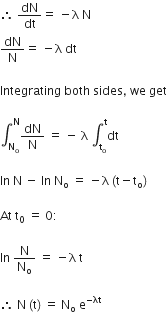
(b)
(i) The β+ decay for is given below:
A proton is converted into neutron if, the unstable nucleus has excess protons than required for stability.
In the process, a positron e+ (or a β+) and a neutrino ν are created and emitted from the nucleus.
p → n + β+ + ν
This process is called beta plus decay.
(ii) The nucleus so formed is an isobar of  because the mass number is same, whereas the atomic numbers are different.
because the mass number is same, whereas the atomic numbers are different.
