Show that the radius of the orbit in hydrogen atom varies as , where n is the principal quantum number of the atom.
The electrostatic force of attraction between the nucleus and the electron is,
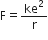
According to Bohr’s theory, a hydrogen atom consists of a nucleus with a positive charge e and a single electron of charge –e, which revolves around it in a circular orbit of radius r.
To keep the electron bound to its orbit, the centripetal force on the electron becomes equal to the electrostatic attraction. Therefore, 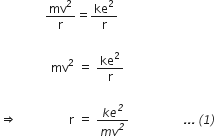
where,
m is the mass of electron, and
v is the speed of an orbit of radius r.
Now, using Bohr’s quantization condition for angular momentum,
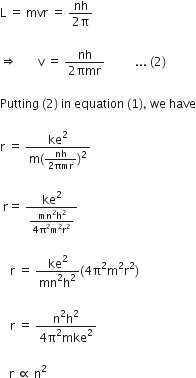
where, n is the principal quantum number.
