(a) Using Bohr’s second postulate of quantization of orbital angular momentum show that the circumference of the electron in the nth orbital state in hydrogen atom is n times the de Broglie wavelength associated with it.
(b) The electron in hydrogen atom is initially in the third excited state.
What is the maximum number of spectral lines which can be emitted when it finally moves to the ground state?i) According to Bohr’s second postulate, we have 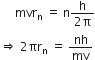
But, as per De- broglie hypothesis
![]()
Therefore, ![]() ; where is the de- broglie wavelength.
; where is the de- broglie wavelength.
ii) Given, electron in the hydrogen atom is in the third excited state.
For third excited state, n = 4
For ground state n= 1
Now, total number of possible spectral lines is given by,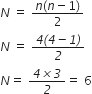
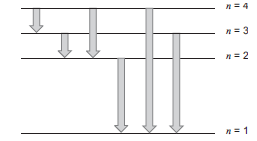
The transition states are as shown in the figure above.
