The energy levels of a hypothetical atom are shown below. Which of the shown transitions will result in the emission of a photon of wavelength 275 nm?
Which of these transitions correspond to emission of radiation of (i) maximum and (ii) minimum wavelength?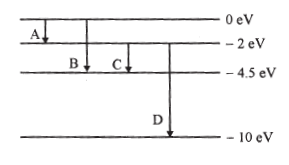
Given, wavelength of the photon,  = 275 nm
= 275 nm
Energy of the photon is given by, 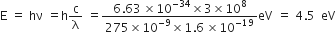
This corresponds to transition B as from the figure.
i) 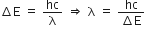
For maximum wavelength should be minimum.
Minimum energy corresponds to transition A.
ii) For minimum wavelength, should be maximum. Maximum energy corresponds to transition D.
