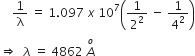The ground state energy of hydrogen atom is – 13.6 eV. If an electron makes a transition from an energy level – 0.85 eV to –3.4 eV, calculate the wavelength of the spectral line emitted. To which series of hydrogen spectrum does this wavelength belong?
Using the formula,![]()
For, n=1; E1 = - 13.6 eV
During the electron transmission, EA = - 0.85 eV to EB = -3.4 eV
So, from equation (i), we have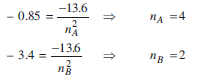
Therefore, electron transition takes place from n=4 to n=2 which corresponds to Balmer series.
We know,![]()
Here,
nA = 4 ; nB = 2 ; R = 1.097 x 107 m-1
Then,