A compound ‘X’ on heating with excess conc. sulphuric acid at 443 K gives an unsaturated compound ‘Y’. ‘X’ also reacts with sodium metal to evolve a colourless gas ‘Z’. Identify ‘X’, ‘Y’ and ‘Z’. Write the equation of the chemical reaction of formation of ‘Y’ and also Write the role of sulphuric acid in the reaction.
'X' on heating with excess conc. sulphuric acid at 443 gives unsaturated compound
An element P (atomic number 20) reacts with an element Q (atomic number 17) to form a compound. Answer the following questions giving the reason :
Write the position of P and Q in the Modern Periodic Table and the molecular formula of the compound formed when P reacts with Q.
Atomic number of element P is 20
electronic configuration = 2, 8, 8, 2
Valence electron =2
Atomic number of element Q is 17
electronic configuration Q = 2, 8, 7
Valence electron = 1
When P reacts with Q, it looses the two valence electrons (valency 2). These two valence electrons are accepted by two Q atoms (valency 1). Hence, the formula of the compound formed between P and Q is PQ2.
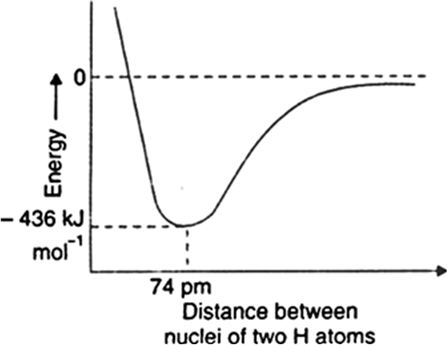
The covalent bond is said to be formed when two atoms achieve stability by sharing of an electron pair, each contributing one electron to the electron-pair. In this way, the atoms can be regarded as having acquired a noble gas configuration.
e.g.,![]()
(a) Why are most carbon compounds poor conductors of electricity?
(b) Write the name and structure of a saturated compound in which the carbon atoms are arranged in a ring. Give the number of single bonds present in this compound.
(a) Carbon compound has covalent bonding and thus it is poor conductors of electricity.
(b) Name - Cyclohexane
Number of the single bond is 18