
Chlorine is prepared in the laboratory by treating manganese dioxide (MnO2) with aqueous hydrochloric acid according to the reaction![]()
How many grams of HCl react with 5.0 g of manganese dioxide?
1 mol [55+2 x16=87 g] MnO2 reacts completely with 4 mol [4x 36.5=146g] of HCl
Therefore,
5.0 g of MnO2 will react with
=(146/87) x5.0g of HCl
=8.4 g of HCl
Hence, 8.4g of HCl will react completely with 5.0g of manganese dioxide.
A welding fuel gas contains carbon and hydrogen only. Burning a small space of it in oxygen gives 3.38 g carbon dioxide, 0·690g of water and no other products. A volume of 10·0L (measured at STP) of this welding gas is found to weigh 11·6g. Calculate: (i) empirical formula (ii) molar mass of the gas and (iii) molecular formula.
i.1mole(44g) of CO2 contains 12g of carbon.
3.38 g of CO2 will contain carbon = (12g/44g) x3.38g =0.9217g
18g of water contains 2g of hydrogen
Therefore 0.690g of water will contain hydrogen=2g/18gx 0.690 =0.0767 g
Since carbon and hydrogen are the only constituents of the compounds, the total mass of the compounds is:
=0.9217+0.767
=0.9984g
Thus,
Percent of C in the compound = (0.9217/.9984) x100 =92.32%
Percent of H in the compound =(0.0767/0.9984) x100=7.68%
Moles of carbon in the compound =92.32/12.00 =7.69
Moles of hydrogen in the compound= 7.68/1=7.68
Since, ration of carbon to hydrogen in the compound=7.69:7.68=1:1
Hence, the empirical formula of the gas is CH.
ii) Given,
Weight of 10.0L of the gas (at S.T.P)=11.6 g
Therefore, weight of 22.4L of gas at STP =(11.6/10.0L) x22.4L
=25.984g
Hence, the molar mass of the gas is 26.0 g.
iii) Empirical formula mass of CH =12+1 =13g
n= molar mass of gas/empirical formula mass of gas
n=26/13
n=2
Therefore, molecular formula of gas= (CH)n
=C2H2
How many significant figures should be present in the answer of the following calculations?![]()
Which one of the following will have largest number of atoms?
C.
1g Li (s)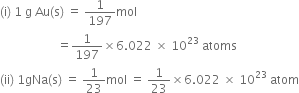
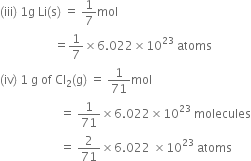
Use the data given in the following table to calculate the molar mass of naturally occurring argon isotopes:
Isotope Isotopic molar mass Abundance
36Ar 35·96755 g mol–1 0·337%
38Ar 37·96272 g mol–1 0·063%
40Ar 39·9624 g mol–1 99.600%
Molar mass of Ar = 35.96755 x 0.00337 + 37.96272 x 0.0063 + 39.9624 x 0.99600
[0.121 +0.024+39.802] = 39.948 g mol-1
