
At 1127 K and 1 atm pressure, a gaseous mixture of CO and CO2 in equilibrium with solid carbon has 90.55% CO by mass
![]()
Calculate Kc for this reaction at the above example.
If the total mass of the mixture of CO and CO2 is 100g. then the mass of CO=90.55g and mass of
CO2= 100-90.55=9-45g
therefore,
Number of moles of CO= 90.55/28=3.23
Number of moles of CO= 9.45/44 =0.22
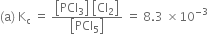
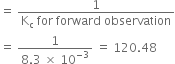
(c)
(i) Kc remains unchanged when more PCl5 is added.
(ii) When pressure is increased, Kc remains unchanged.
(iii) As given reaction is endothermic, on increasing the temperature, Af will increase.
As 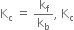 will increase with the increase of temperature.
will increase with the increase of temperature.
(a) 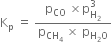
(b)
(i) The value of Kp remains unchanged on increasing the pressure. According to Le Chatelier’s principle, equilibrium will shift in the backward direction.
(ii) In case of endothermic reactions the value of increases with the increase in temperature. According to Le Chatelier principle, equilibrium will shift in the forward direction.
(iii) Kp will remain undisturbed i.e. equilibrium composition will not be disturbed but equilibrium will be attained equally.However, in the presence of a catalyst, the equilibrium would be attained quickly.
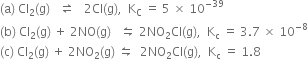
Describe the effect of:
(a) addition of H2
(b) addition of CH3OH
(c) removal of CO
(d) removal of CH3OH
on the equilibrium of the reaction:
![]()
(a) According to Le-Chatelier’s principle, equilibrium will shift in the forward direction.
(b) According to Le-Chatelier’s principle, equilibrium will shift in the backward direction.
(c) According to Le-Chatelier’s principle, equilibrium will shift in the backward direction.
(d) According to Le-Chatelier’s principle, equilibrium will shift in the forward direction.
