The angular momentum of electrons in d orbital is equal to




A.

Angular momentum of electrons in d- orbital is 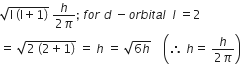
Magnetic moment 2.84 BM is given by (At. no. Ni= 28, Ti= 22, Cr=24, Co = 27)
Ni2+
Ti3+
Cr3+
Cr3+
A.
Ni2+
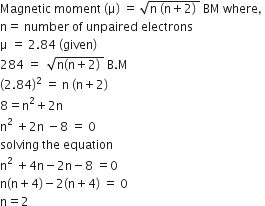
The ratio of the shortest wavelength of two spectral series of hydrogen spectrum is found to be about 9. The spectral series are:
Paschen and Pfund
Lyman and Paschen
Brackett and Pfund
Balmer and Brackett
B.
Lyman and Paschen
Shortest wavelength of lymen series:
Two electrons occupying the same orbital are distinguished by
Magnetic quantum number
Azimuthal quantum number
Spin quantum number
Spin quantum number
C.
Spin quantum number
Two electrons occupying the same orbital has equal spin but the directions of their spin are opposite. Hence, spin quantum number, s(represented +1/2 and -1/2) distinguishes them.
What is the maximum number of orbitals that can be identified with the following quantum numbers?
n=3, l =1, m1 = 0
1
2
3
3
A.
1
The value of n=3 and l =1 suggest that it is a 3p orbital while the value of m1 = 0 [magnetic quantum number] shows that the given 3p orbital is 3pz in nature.
Hence, the maximum number of orbitals identified by the given quantum number is only 1, i.e. 3pz.
