The ratio of masses of oxygen and nitrogen of a particular gaseous mixture is 1:4. The ratio of number of their molecule is
1:4
7:32
1:8
1:8
B.
7:32
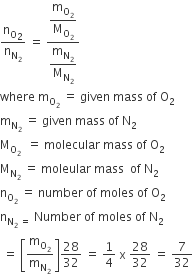
3 g of activated charcoal was added to 50 mL of acetic acid solution (0.06N) in a flask. After an hour it was filtered and the strength of the filtrate was found to be 0.042 N. The amount of acetic acid adsorbed (per gram of charcoal) is:
18 mg
36 mg
42 mg
42 mg
A.
18 mg
The initial strength of acetic acid = 0.06N
Final strength = 0.042 N
Volume given = 50 mL
there Initial m moles of CH3COOH
= 0.06 x 50 = 3
Final m moles of CH3COOH
= 0.042 x 50 = 21
therefore, m moles of CH3COOH absorbed
= 3-2.1
= 0.9 m mol
Hence, mass of CH3COOH absorbed per gram of charcoal
= 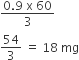
The vapour pressure of acetone at 20oC is 185 torr. When 1.2 g of a non-volatile substance was dissolved in 100 g of acetone at 20oC, its vapour pressure was 183 torr. The molar mass (g mol-1 ) of the substance is:
32
64
128
128
B.
64
Given,
po = 185 torr at 20oC
ps = 183 torr at 20oC
Mass of non-volatile substance,
m= 1.2 g
Mass of acetone taken = 100 g
As we have,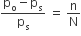
putting the values, we get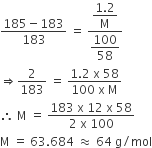
For the estimation of nitrogen 1.4g of an organic compound was digested by Kjeldahl's method and the evolved ammonia was absorbed in 60 mL of M/10 sulphuric acid. The unreacted acid required 20 mL of M/10 sodium hydroxide for complete neutralisation. The percentage of nitrogen in the compound is
6%
10%
3%
3%
B.
10%
Consider separate solution of 0.500 M C2H5OH (aq), 0.100 M Mg3(PO4)2 (aq) 0.250 M KBr (aq) and 0.125 M Na3PO4 (aq) at 25oC. Which statement is true about these solutions, assuming all salts to be strong electrolytes?
They all have the same osmotic pressure
0.100 M Mg3(PO4)2 (aq) has the highest osmotic pressure
0.125 M Na3PO (aq) has the highest osmotic pressure
0.125 M Na3PO (aq) has the highest osmotic pressure
A.
They all have the same osmotic pressure
effective molarity = Van't Hoff factor x molarity
0.5 M C2H5OH (aq) i =1
Effective molarity = 0.5
0.25 M KBr (aq) i = 2
Effective molarity = 0.5
0.1 M Mg3(PO4)2 (aq) i = 5
Effective molarity = 0.5 M
0.125 M Na3PO4 (aq)
Effective molarity = 0.5 M
Hence, all colligative properties are same
