Answer:
Activation of adsorbent implies increasing its adsorbing power. It is achieved by the following ways:
(i) Activation of the adsorbent is carried out by mechanical rubbing or by subjecting it to some chemical reaction.
(ii) Some adsorbents are activated by strong heating in contact with super-heated steam. For example, charcoal which is commonly employed in adsorption of gases, is activated by subjecting it to the action of superheated steam. This will remove the molecules of gases like CH4, C2H6 (hydrocarbons) that are occupying these pores.
(iii) To increase the adsorbing power, the adsorbent is sub divided into small fine pieces. By doing so, the surface area increases and so is the adsorbing power.
Answer:
Adsorption plays an important role in heterogeneous catalysis. Adsorption is surface phenomenon in which the substances (reactants) get concentrated only on the surfaces of solid adsorbents and do not penetrate into it.This increases concentration of reactants and there is a great probability of occurrence of reaction. The catalyst adsorbs reactants and forms a intermediate compound known as activated complex. Following are various ways in which catalytic adsorption increases the reaction rate:
(i) The concentration of reactants on surface of solid adsorbent increases and it becomes easier for molecules to attack each other for effective collisions, forming products.
(ii) A particular part of reactant molecule may come in contact with other molecules which otherwise is difficult.
(iii) Some adsorbed molecules dissociate into atoms or free radicals which are very reactive and thus increase the reaction rate.
(iv) Heat of adsorption released provides activation energy for formation of activated complex and then increase the rate of reaction.
Answer:
Adsorption is always exothermic, this statement can be explained by two.
i) Adsorption leads to a decrease in the residual forces on the surface of the adsorption.
This cause a decrease in the surface energy of the adsorption therefore adsorption is always exothermic.
ii) ΔH of adsorption is always negtive.
When a gas is adsorbed on a soild surface its movement is restricted leading to decrease in the entropy of the gas i.e. ΔS
Adsorption is accompanised by decrease in entropy, i.e., ΔS = – ve. If it is spontaneous then ΔG should be negative.
ΔG = ΔH – TΔS
Since ΔS is – ve, ΔG will be – ve only if
ΔH = – ve, i.e., if process is exothermic.
Answer:
The factors which influence the adsorption of a gas on a solid are:
(1) The nature of a gas adsorbate and nature of solid adsorbent.
(2) Surface area of adsorbent.
(3) Pressure of gas.
(4) Temperature.
(5) Activation of adsorbent.
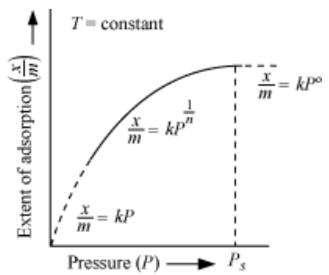
At low pressure, the extent of adsorption is directly proportional to pressure (raised to power one).
![]()
At high pressure, the extent of adsorption is independent of pressure (raised to power zero).
![]()
Therefore at an intermediate value of pressure, adsorption is directly proportional to pressure raised to power 1/n. Here n is a variable whose value is greater than one.
![]()
Using constant of proportionality, k, also known as adsorption constant we get
![]()
The above equation is known as Freundlich adsorption equation.
As per Freundlich adsorption equation
![]()
The equation above equation is comparable with comparable with the equation of a straight line,
y = m x + c where m represents the slope of the line and c represents intercept on y-axis.
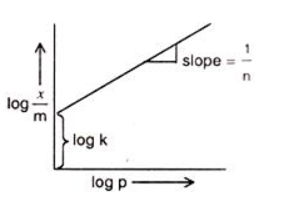
Plotting a graph between log(x/m) and log p, we will get a straight line with the value of slope equal to 1/n and log k as y-axis intercept.