In the Hofmann bromamide degradation reaction, the number of moles of NaOH and Br2 used per mole of amine produced are:
Four moles of NaOH and two moles of Br2
Two moles of NaOH and two moles of Br2
Four moles of NaOH and one mole of Br2
Four moles of NaOH and one mole of Br2
C.
Four moles of NaOH and one mole of Br2
Hofmann-bromamide degradation reaction is given as
RCONH2 + 4 NaOH + Br2 → RNH2 + Na2CO3 + 2NaBr + 2H2O
Hence four moles of NaOH and one mole of Br2 are used.
A compound with molecular mass 180 is acylated with CH3COCl to get a compound with molecular mass 390. The number of amino groups presents per molecule of the former compound is
2
5
4
4
B.
5
On heating an aliphatic primary amine with chloroform and ethanolic potassium hydroxide, the organic compound formed is
an alkanol
an alkaneodiol
an alkyl cyanide
an alkyl cyanide
D.
an alkyl cyanide
The reaction is an example of carbylamines reaction which includes conversion of amine to isocyanide.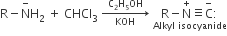
Considering the basic strength of amines in aqueous solution, which one has the smallest pKb value?
(CH3)2NH
CH3NH2
(CH3)3N
(CH3)3N
A.
(CH3)2NH
Order of basic strength of aliphatic amine in aqueous solution is as follows (order of Kb)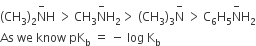
So (CH3)2 NH will have smallest pKb value.
In the case of phenylamine, N is attached to sp2 hybridised carbon, hence it has highest pKb and least basic strength.
