
Corrosion is the process of slowly eating away of the metal due to attack of the atmospheric gases on the surface of the metal resulting into the formation of compounds such as oxides, sulphides, carbonates, etc.
The rusting of iron, tarnishing of silver, development of green coating on copper and bronze are some of the examples of corrosion.
The main factors which affect corrosion are
Presence of water and the electrolytes present in it.
1. More the reactivity of metal, the more will be the possibility of the metal getting corroded.
2. The impurities help in setting up voltaic cells, which increase the speed of corrosion
3. Presence of electrolytes in water also increases the rate of corrosion
4. Presence of CO2 in natural water increase rusting of iron.
5. When the iron surface is coated with layers of metals more active than iron, then the rate of corrosion is retarded.
6. A rise in temperature (with in a reasonable limit) increases the rate of corrosion.
(i) Cathodic protection (CP) is a technique used to control the corrosion of a metal surface by making it the cathode of an electrochemical cell. A simple method of protection connects the metal to be protected to a more easily corroded sacrificial metal to act as the anode.
for example zinc is used to prevent iron
Zinc is more electro-positive than iron. Therefore, as long as zinc is there on the iron pipe, zinc acts as anode and the iron as cathode. As a result, rusting of iron is prevented.
(ii)Electrochemical series is a series of chemical elements arranged in order of their standard electrode potentials. The hydrogen electrode. H+(aq) + e- →← 1/2H2(g) is taken as having zero electrode potential. An electrode potential is, by definition, a reduction potential
(iii)The quantity 1/A is called cell constant denoted by the symbol. G*. It depends on the distance between the electrodes and their area of cross -section and has the dimension of length-1 and can be calculated if l and A
G* =l/A =Rk
(iv) A strong electrolyte is a solute that completely, or almost completely, ionizes or dissociates in a solution. While the specific conductance of a solution increases with concentration, the equivalent conductance decreases as the concentration increases. unit of equivalent conductance
(v)electrolytes :A substance that when dissolved in water produced a solution that can conduct electric current.
there are two electrolytes
1. strong
2.weak
strong Electrolytes conduct current very efficiently. Completely ionized or dissociate when dissolved in water
a. Soluble Ionic compounds
b. Strong acids (HNO3(aq), H2SO4(aq), HCl(aq))
HNO3--> H+ + NO3- (100% ionization)
c. Strong bases (KOH and NaOH)
KOH -->K+ +OH - (100% dissociation)
Weak electrolytes conduct only a small current
Slightly ionized in solution
a. Weak acids (organic acids-->acetic, citric, butyric,malic, etc.)
HC2H3O2 <==> H+ + C2H3O2-
b. Weak bases (ammonia)
NH3 + H2O <==> NH4+ + OH-
If we connect the zinc and copper by means of a metallic conductor, the excess electrons that remain when Zn2+ ions emerge from the zinc in the left cell would be able to flow through the external circuit and into the right electrode, where they could be delivered to the Cu2+ ions which become "discharged", that is, converted into Cu atoms at the surface of the copper electrode. The net reaction is the oxidation of zinc by copper(II) ions:
but this time, the oxidation and reduction steps (half reactions) take place in separate locations:
left electrode : Zn----> Zn2+ +2e-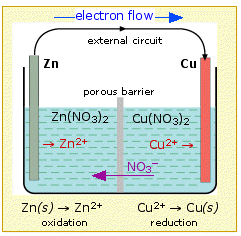
| galvanic cell | Electrolytic cell |
|---|---|
| A Galvanic cell converts chemical energy into electrical energy. | An electrolytic cell converts electrical energy into chemical energy. |
| Here, the redox reaction is spontaneous and is responsible for the production of electrical energy. | The redox reaction is not spontaneous and electrical energy has to be supplied to initiate the reaction. |
| The two half-cells are set up in different containers, being connected through the salt bridge or porous partition. | Both the electrodes are placed in a same container in the solution of molten electrolyte. |
| Here the anode is negative and cathode is the positive electrode. The reaction at the anode is oxidation and that at the cathode is reduction. | Here, the anode is positive and cathode is the negative electrode. The reaction at the anode is oxidation and that at the cathode is reduction. |
| The electrons are supplied by the species getting oxidized. They move from anode to the cathode in the external circuit. | The external battery supplies the electrons. They enter through the cathode and come out through the anode. |
a)
|
electrolytes |
nonelectrolyte |
|
An electrolyte dissociates in solution and thus produce ion.
|
A nonelectrolyte does not dissociate at all in solution and therefore does not produce any ions. |
|
Electrolytes are ionic substance that dissolve in water |
Nonelectrolytes are typically polar covalent substances that do dissolve in water as molecules instead of ions. |
C)
|
Primary cell |
Secondary |
|
Lower initial cost. |
Higher Initial Cost |
|
Higher life-cycle cost ($/kWh). |
Lower life-cycle cost ($/kWh) if charging in convenient and inexpensive |
|
Disposable. |
Regular maintenance required. |
|
Typically lighter and smaller thus traditionally more suited for portable applications. |
Traditionally less suited for portable applications, although recent advances in Lithium battery technology have lead to the development of smaller/lighter secondary batteries. |
|
Molar conductivity |
Specific conductivity |
|
Molar Conductivity of a solution at a given concentration is the conductance of the volume V of solution containing one mole of electrolyte kept between two electrodes with area of cross section A and distance of unit length. Therefore, Distance is unit so l = 1 Volume = area of base × length So V = A × 1 = A Λm =κA/l Λm = κV
|
Conductivity of a solution is equal to the conductance of a solution of 1 cm length and cross section area of 1 square cm. it may also be define as the conductance of ine centimeter cube of the conductor . It is represented by the symbol Kappa (κ). mathematically we can write κ = 1/ p here ρ is resistivity the unit of K is ohm –1 cm –1 or S cm–1 The conductivity, κ, of an electrolytic solution depends on the concentration of the electrolyte, nature of solvent and temperature.
|
