Answer:
When a solution does not obey Raoult’s law over the entire range of concentration, then it is called non-ideal solution.
The vapour pressure of such solution either be higher or lower. i.e positive when higher
negtive when lower.
The cause for these deviations lie in the nature of interactions at the molecular level.
In case of positive deviation from Raoult’s law,
A-B interactions are weaker than those between A-A or B-B, i.e., in this case the intermolecular attractive forces between the solute-solvent molecules are weaker than those between the solute-solute and solvent-solvent molecules. This means that in such solutions, molecules of A (or B) will find it easier to escape than in pure state. This will increase the vapour pressure and result in positive deviation. Mixtures of ethanol and acetone behave in this manner.
In case of negative deviations from Raoult’s law, the intermolecular attractive forces between A-A and B-B are weaker than those between
A-B and leads to decrease in vapour pressure resulting in negative deviations. An example of this type is a mixture of phenol and aniline.
In this case the intermolecular hydrogen bonding between phenolic proton and lone pair on nitrogen atom of aniline is stronger than the
respective intermolecular hydrogen bonding between similar molecules.
Answer:
Raoult's law states that for a solution of volatile liquid, the partial vapour pressure of each component in the solution is dirctly proportional to its mole fraction.
Thus if there is solution of two liquid component 1 and 2 then,
for component 1,
Answer:
A molar mass that is either lower or higher than the expected or normal value is called as abnormal molar mass.
In order to account for all such abnormalities, introduced a factor
(i) known as van 't Hoff's factor, which represents the extent of association (or) dissociation of a solute.
Van't Hoff's factor is defined as the ratio of the observed colligative property to the calculated colligative property.
i = Observed colligative property / Calculated colligative property
Observed colligative property ∝ 1/Molar Mass
i = Mc/Mo
Van't Hoff's factor
(i) represents the extent of association (or) dissociation of a solute
i = Total number of moles of particles after association or dissociation / Number of moles of particles before association or disscussion
Experimentally determined molar mass is always lower than actual value for dissociation.
Molar Mass ∝ 1/Colligative Property
If the solute undergoes association in a solution, then the value of van 't Hoff's factor is less than one. If the solute undergoes dissociation then 'i' is greater than one.
Ex:
KCl → K+ + Cl-
1 molecule of KCl furnishes 2 ions in solution
i = Total number of moles of particles after dissociation / Number of moles of particles before dissociation
i = 2/1 = 2
2CH3COOH ⇔(CH3COOH)2
Ethanoic acid Dimer of Ethanoic acid
i = Total number of moles of particles after association / Number of moles of particles before association
i = 1/2 = 0.5
The solution of ethanol and cyclohexane show positive deviation.
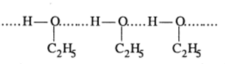
Another case can be, the mixture of chloroform and acetone shows negtive deviation. When these are mixed , hydrogen bond is formed and negative deviation shown;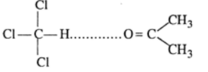
State with a suitable diagram and appropriate examples why some non-ideal solutions. Show positive deviation from ideal behaviour.
In case of negative deviations from Raoult's law, the intermolecular attractive forces between A-A and B-B are weaker than those between A-B and leads to decrease in vapour pressure resulting in negative deviations. An example of this type is a mixture of phenol and aniline.
In this case the intermolecular hydrogen bonding between phenolic proton and lone pair on nitrogen atom of aniline is stronger than the respective intermolecular hydrogen bonding between similar molecules.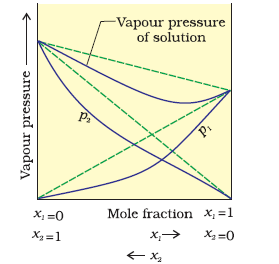
This decreases the escaping tendency of molecules for each component and consequently the vapour pressure decreases resulting in negative deviation from Raoult's law
