What is a fractionating column? Explain its advantage.
When the boiling points of the two liquids are quite close (difference less than 20-25°C) the separation is not successfully affected by using simple distillation process but is affected by fitting the flask with a fractionating column.
A fractionating column is a long tube provided with obstructions or packed with glass beads. The obstructions provided slows down the passage of vapours upwards and that of the liquid downwards.
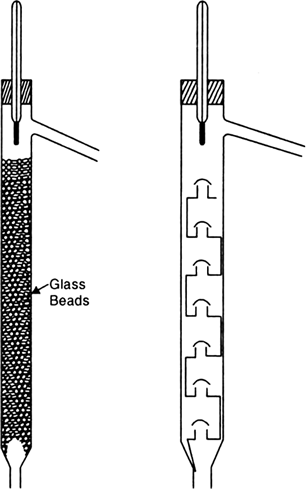
Advantages. Suppose you have a mixture of liquids (say A with boiling point 80°C and B with boiling point 90°C). Set up the apparatus as shown in Fig. 2.9. On heating the flask the vapours obtained consist more of A and less of B. As the vapours rise up the fractionating column, they condense partially and the condensed liquid flows down. Vapours of B having higher boiling point will condense more rapidly than the vapours of A having lower boiling point. As a result the vapours moving up get richer in A. The condensed liquid flowing down meets the ascending stream of vapours and in the process takes away more of high boiling liquid B. The process is
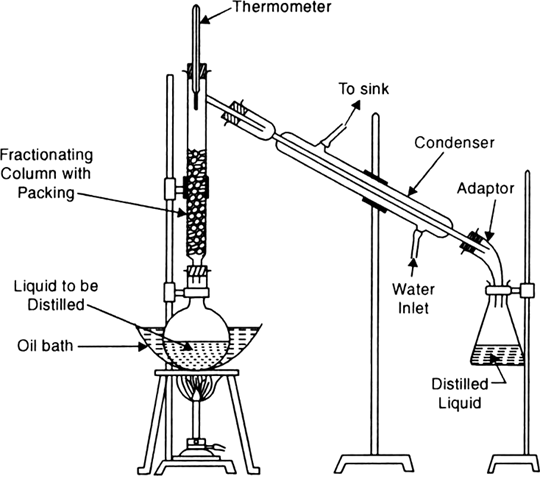
repeated throughout the length of fractionating column. Consequently, the vapours which escape from the top of the fractionating column into the condenser consist of almost pure A. Thus the distillate received is pure A whereas the liquid left behind in the flask is rich in B. Pure B can then be obtained by simple distillation. The process of fractional distillation with fractions A and B are repeated if the boiling point difference is very low.
You are given a mixture of sand, water and mustard oil. How will you separate the components of the mixture.
Take the mixture of sand, water and mustard oil. Let the solution to stand undisturbed for about half an hour. The heavy sand particles settle down at the bottom of the container. Now transfer the liquid to another beaker in such a way that the sand remains in the first beaker.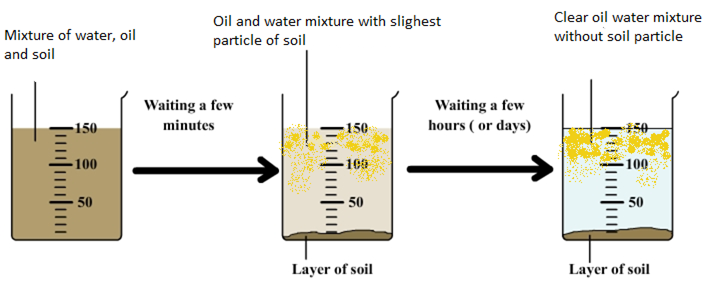
Take the remaining mixture of mustard oil and water in a separating funnel. Let the funnel to stand undisturbed for half an hour. Water and mustard oil forms two distinct layers. Oil being lighter forms upper layer while the water forms lower layer. Open the tap of the separating funnel and let the lower layer to collect down in the beaker. Close the tap when lower water layer is completely separated. Take out the oil layer in another beaker. Thus the components of mixture are separated.
Name the processes for separation in the following cases:
(i) Fine insoluble particles suspended in a liquid.
(ii) A solid substance dissolved in a liquid.
(iii) A sublimable solid mixed with other solids.
(iv) Salt from sea water.
(v) Three solid substance soluble in a mixture of solvents.
(vi) Two liquids which are completely immiscible.
(vii) Two miscible liquids having 10°C difference in their boiling points.
(viii) Mixture of nitrogen and helium gases.
(i) Centrifugation and filtration or decantation.
(ii) Evaporation or distillation or crystallization.
(iii) Sublimation.
(iv) Evaporation or crystallization.
(v) Chromatography.
(vi) Separating funnel.
(vii) Fractional distillation.
(viii) Cooling and fractional distillation.
Give reason for each of the following:
(i) Ripening of a fruit is a chemical change.
(ii) Crystallization of a substance from its solution is a physical change.
(iii) Formation of curd from milk is a chemical change.
(iv) Separation of gases from air is a physical change.
(i) Composition and properties of fruit initially are entirely different than those after ripening of fruit. It cannot be reversed. Hence, it is a chemical change.
(ii) Crystallization of a substance is a physical change because there is no change in composition and chemical properties of the substance. The crystallized substance can again be made into solution.
(iii) Curd and milk have entirely different properties and curd cannot be changed to milk. Hence it is a chemical change.
(iv) Separation of gases from air is a physical change because separated gases can again be mixed.
Classify the following as a chemical or physical change.
(i) Cutting of trees; (ii) Melting of butter in a pan; (iii) An almirah gets rusted; (iv) Water boils to form steam; (v) Electric current is passed through water and it is broken down into hydrogen and oxygen gas; (vi) Dissolving salt in water.
(i) Cutting of trees–Physical Change
(ii) Melting of butter in a pan–Physical change
(iii) An almirah gets rusted–Chemical change
(iv) Water boils to form steam–Physical change
(v) Electric current is passed through water and it is broken down into hydrogen and oxygen–Chemical change
(vi) Dissolving salt in water–Physical change
