 Short Answer Type
Short Answer Type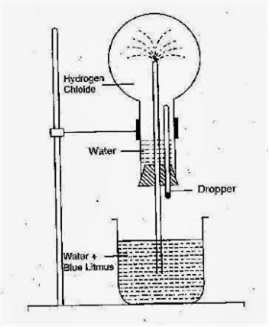
i) Fountain Experiment
ii) Hydrogen chloride is soluble in water
iii) Red.
A, B, C and D summarise the properties of Sulphuric acid depending on whether it is dilute or concentrated. Choose the property (A, B, C or D. depending on which is relevant to each of the preparations (i) to (iii) :-
A. Dilute acid (typical acid properties).
B. Non-volatile acid.
C. Oxidising agent.
D. Dehydrating agent.
i) Preparation of Hydrogen chloride
ii) Preparation of ethene from Ethanol.
iii) Preparation of Copper sulphates from copper oxide.
 Long Answer Type
Long Answer TypeThe volume of gases A,B,C and D are in the ration, 1:2:2:4 under the same conditions of temperature of pressure.
i) which sample of gas contain the maximum number of molecules?
ii) If the temperature and the pressure of gas A are kept constant, then what will happen to the volume of A when the number of molecules is doubled.
iii) If this ratio of gas volumes refers to the reactant and products of a reaction, which gas law is being observed?
iv) If the volume of A is actually 5.6 dm3 at S.T.P., calculate the number of molecules in the actual volume of D at S.T.P., (Avogadro's Number is 6 x 1023)
v) Using your answer from (iv), state the mass of D if the gas is dinitrogen oxide (N2O),(N= 14; O=16).
In the manufacture of Iron, a mixture of Limestones, Coke and Iron ore is added to the blast furnace. In this context:-
i) State the purpose od adding Limestone to the furnace.
ii) Give the equation for the reduction of the Iron ore.
iii) Name the substance which is collected along with Cast iron at the bottom of the furnace.
iv) Write the chemical equations for the formation of the substance named in (iii) above.
