 Long Answer Type
Long Answer TypeThe following is an extract from metals in the services of man alexander and street/pelican 1976:
Alumina (aluminium oxide) has a very high melting point of over 20000C so that it cannot readily be liquefied. However, conversion of alumina to aluminium and oxygen, by electrolysis, can occur when it is dissolved in some other substance.
(I)  Which solution is used to react with bauxite as a first step in obtaining a pure aluminium oxide? 
(II) The aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide. Write the equation for this reaction.
(III) Name the element which serves both as the anode and the cathode in the extraction of aluminium
(IV) Write the equation for the reaction that occurs at the cathode during the extraction of aluminium by electrolysis
(V) Give the equation for the reaction which occurs at the anode when aluminium is purified by electrolysis.
i) Sodium hydroxide solution
ii) 2Al(OH)3+heat --->  Al2O3 +3H2O
iii) Carbon serves both as the anode and the cathode in the extraction of aluminium.
iv) Al3+ +3e------> Al
v) Al---->  Al3+ +3e-
Q2 (a) some properties of sulphuric acid are listed below. Choose the property A,B, C and D which is responsible for the reaction (i) to (v) some properties may be repeated:
A)     Acid
B)      Dehydrating agent
C)      Non-volatile acid
D)     Oxidizing agent
(i)    C12H22O11 +2H2SO4 ---> 12C +11H2O +nH2SO4 
(ii)    S+2H2SO4 ----> 3SO2 +2H2O 
(iii)   NaCl +H2SO4 ---> NaHSO4 +HCl.
(iv)  CuO +H2SO­4 ---> CuSO4 +H2O 
(v)   Na2CO3 +H2SO4 ----> Na2SO4 +H2O +CO2
Give the IUPAC names of the following compounds numbered (i) to (v). The IUPAC names of the compounds on the left are to guide  you into giving the correct IUPAC names of the compounds on the right.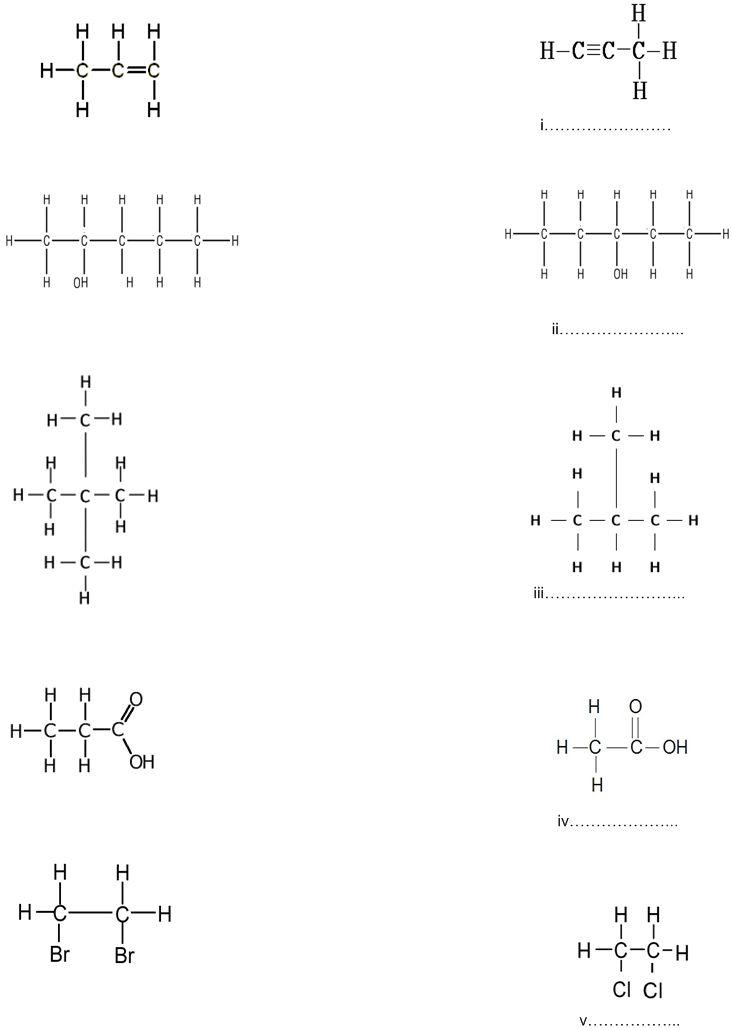
Copy and complete the following table which relates to three homologous series of hydrocarbon:
|
General Formula |
CnH2n |
CnH2n-2 |
CnH2n+2 |
|
IUPAC name of the homologous series |
  |
  |   |
|
Characteristic bond tyres |
  |
  |   |
|
IUPAC name of first member of the series |
  |
  |   |
|
Types of reaction with chlorine |
  |
  |   |
