 Short Answer Type
Short Answer TypeWrite the equation for the following reaction:
Dilute nitric acid and copper:
3Cu +8HNO3 ----> 3Cu(NO3)2 +2NO +4H2O
What property of hydrogen chloride is demonstrated when it is collected by downward delivery(upward displacement)?
Dilute hydrochloric acid and sodium thiosulphate
Write the equation for the following reaction:
Dilute hydrochloric acid and lead nitrate solution.
Here is an electrode reaction
Cu---> Cu2+ +2e-
At which electrode (anode or cathode) would such a reaction take place? Is this an example of oxidation or reduction ?
A solution contains magnesium ions (Mg2+) iron (II) ions (Fe2+) and copper ions (Cu2+). On passing an electric current through this solution which ions will be the first to be discharged at the cathode? Write the equation for the cathode reaction.
The following is a sketch of an electrolytic cell used in the extraction of aluminium:
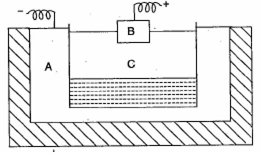
(i) What is the substance of which the electrodes A and B made?
(ii) At which electrode (A and B) is the aluminium formed?
(iii) What are the two aluminium compounds in the electrolyte C?
(iv) Why is it necessary for electrode B to be continuously replaced?
From the equation:
C+2H2SO4 ----> CO2 +2H2O +2SO2
Calculate :
i) The mass of carbon oxidised by 49 g of sulphuric acid (C=12; relative molecular mass of sulphuric acid =98)
ii)The volume of sulphur dioxide measured at STP, liberated at the same time (volume occupied by 1 mole of a gas at STP is 22.4 dm3
