 Short Answer Type
Short Answer TypeGiven the equation for the preparation of each of the following salts from the starting material given:
Iron (III )chloride from iron.
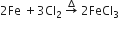
given the equation for the preparation of each of the following salts from the starting material given:
Potassium sulphates from potassium hydroxide solution.
 Long Answer Type
Long Answer TypeCompound A is bubbled through bromine dissolved in carbon tetrachloride and the product is CH2Br-CH2Br.
A  CH2Br-CH2Br
CH2Br-CH2Br
i) Draw the structural formula of A.
ii) What type of reaction has A undergone?
iii)What is your observation?
iv)Name (not formula) the compound formed when steam reacts with A in the presence of phosphoric acid.
v) What is the procedure for converting the product of (b) (iv) back to A?
4.5 moles of calcium carbonate are reacted with dilute hydrochloric acid.
i) Write the equation for the reaction.
ii) What is the mass of 4.5 moles of calcium carbonate? (Relative molecular mass of calcium carbonate is 100).
iii)What is the volume of carbon dioxide liberated at stp?
iv)What mass of calcium chloride is formed? (Relative molecular mass of calcium chloride is 111.)
v) How many moles of HCl are used in this reaction?
Mr. Ramu wants to electroplate his key chain with nickel to prevent rusting, For this electroplating:
(i) Name the electrolyte
(ii) name the cathode.
(iii) name the anode
(iv) give the reaction at the anode
(v ) give the reaction at the anode.
The diagram shows an apparatus for the laboratory preparation of hydrogen chloride,
Structure.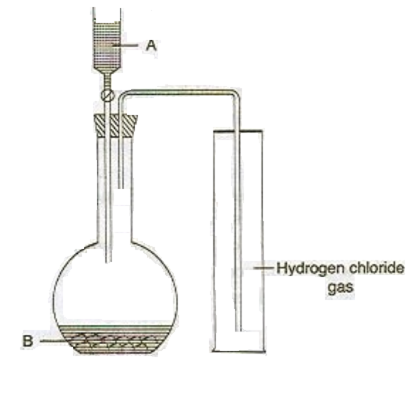
(i) Identify A and B.
(ii) Write the equation for the reaction.
(iii) How would you check whether or not the gas jar is filled with hydrogen chloride?
(iv) What does the method of collection tell you about the density of hydrogen chloride?
