 Short Answer Type
Short Answer TypeGive a chemical equation for the reaction between ethyl alcohol and acetic acid.
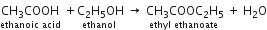
 Long Answer Type
Long Answer TypeConcentrated nitric acid oxidised phosphorus to phosphoric acid according to the following equation:
P+5HNO3(conc.)---> H3PO4 +H2O +5NO2
If 9.3 g of phosphorus was used in the reaction, calculate:
I) Number of moles of phosphorus taken.
II) The mass of phosphoric acid formed.
III)The volume of nitrogen dioxide produced at STP.
[H=1, N=14,P=31,O=16]
i) 67.2 litres of hydrogen combines with 44.8 litres of nitrogen to form ammonia under specific conditions as:
N2(g) +3H2(g) --> 2NH3(g)
Calculate the volume of ammonia produced. What is the other substance, if any that remains in the resultant mixture?
ii)The mass of 5.6 dm3 of a certain gas at STP is 12.0g calculate the te relative molecular mass of the gas.
iii) Find the total percentage of Magnesium in magnesium nitrate crystals Mg(NO3)2.6H2O.
[Mg =24; N=14; O=16 and H =1]
Some word/words are missing in the following statements. You are required to rewrite the statement in the correct form using the appropriate word/words:
Cations migrate during electrolysis.
Refer to the flow chart diagram below and give balanced equation with condition any, for the following conversion A to D.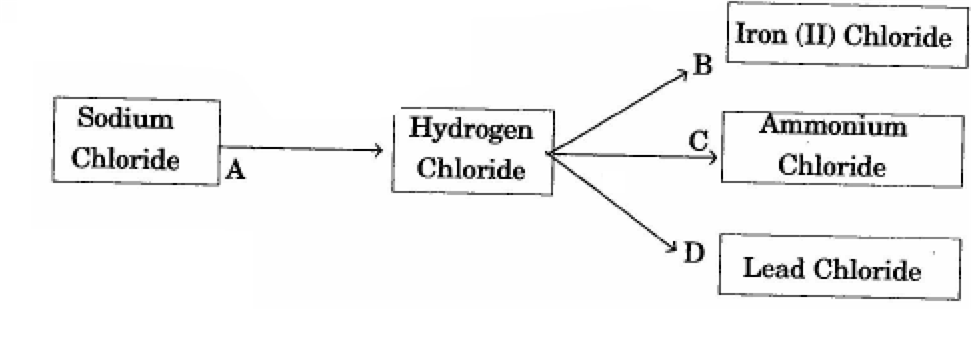
Q6. (c ) the following question is based on the preparation of ammonia gas in the laboratory:
I) Explain why ammonium nitrate is not used in the preparation of ammonia.
II) Name the compound normally used as a drying agent during the process.
III) How is ammonia gas is collected?
IV) Explain why it is not collected over water.
Q2. (a) some properties of sulphuric acid are listed below. Choose the role played by sulphuric acid as A, B,C and D which is responsible for the reaction (i) to (v) some role/s may Be repeated.
A) Dilute acid
B) Dehydrating agent
C) Non-volatile acid
D) Oxidising agent
i) CuSO.5H2 (conc. H2SO4) ---> CuSO4 +5H2O
ii) S+H2SO4 (conc.) ----> 3SO2 +2H2O
iii) NaNO2 +H2SO4 (conc.) NaHSO4 +HCl
NaHSO4 +HCl
iv) MgO +H2SO4 -----> MgSO4 +H2O
v) Zn+2H2SO4-----> ZnSO4 +SO2 +2H2O
Give the structural formula for the following:
i) Methonic acid
ii) Ethanol
iii)Ethyne
iv)Acetone
v)2-methyl propane.
From the following organic compounds given below, choose one compound in each case which relates to the description i) to iv)
[ethyne,ethanol,acetic acid,ethane,methane]
i) An unsaturated hydrocarbon used for welding purposes.
ii) An organic compound whose functional group is carboxyl
iii) A hydrocarbon which on catalytic hydrogenation gives a saturated hydrocarbon.
iv) An organic compounds used as a thermometric liquid.
