 Short Answer Type
Short Answer Type(i) State the two necessary conditions for a compound to show geometrical isomerism.
(ii) Why is meso-tartaric acid optically inactive ?
(iii) Write the structure of three compounds which have the same molecular formula of C4H8O but have different functional groups.
(i) Necessary condition for geometrical isomerism.
(i) Molecules must contain a double bond.
(ii) Each of two carbon atoms of double bond must have different substituents, which may be same or different.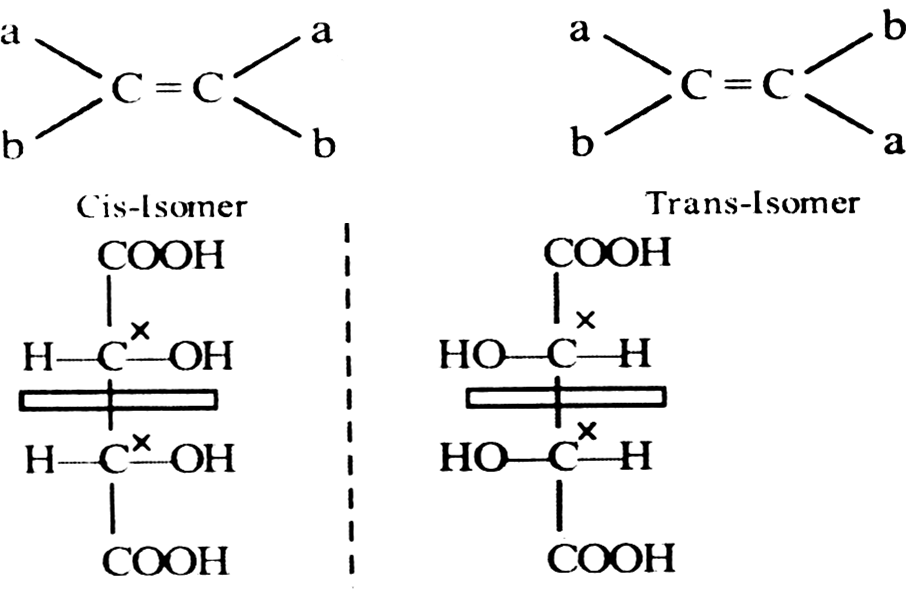
Meso-tartaric acid optically inactive. Because it has a plane of symmetry does not enantiomerism plane symmetry divide the molecule into two equal halves, so that one half is mirror image of other.
(i) Give any one example of a cross-linked synthetic polymer.
(ii) With reference to the polymer named by you :
(1) Write the compounds from which it is prepared.
(2) Give one physical property of the cross-linked synthetic polymer.
 Long Answer Type
Long Answer TypeWrite the relevant equations to convert:
(i) Fructose to osazone.
(ii) Acetic acid to acetone.
(iii) Acetyl chloride to acetic anhydride.
Give one test each to distinguish between :
(i) Acetaldehyde and formaldehyde.
(ii) Acetone and acetic acid.
(iii) Starch and cellulose.
(i) Benzaldehyde reacts with an alcoholic solution of potassium cyanide.
(ii) Aniline is warmed with chloroform and alcoholic potassium hydroxide.
(iii) Propanone is treated with iodine and excess of alkali and warmed.
(iv) Benzoic acid is treated with soda lime.
