 Short Answer Type
Short Answer TypeAnswer the following:
Write the reaction to prepare acetaldehyde from hydrogen gas and an acid chloride.
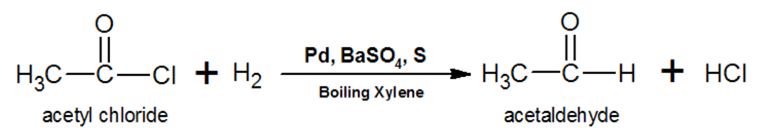
A 10% aqueous solution of cane sugar (mol. wt 342) is isotonic with 1.754% aqueous solution of urea. Find the molecular mass of urea.
Determine the pH value of 0.001 M acetic acid solution if it is 2% ionised at this concentration. How can the degree of dissociation of this acetic acid solution be increased?
Sulphur dioxide acts as an oxidizing agent as well as a reducing agent. Give one reaction to show its oxidizing nature and its reducing nature.
an organic compound A has the molecular formula C7H6O. When A is treated with NaoH followed by acid hydrolysis, it gives two products B and C. When B is oxidized it gives A, When A and C are each treated separately with PCl5, they give two different products D and E.
i) Identify A,B,C,D and E.
ii) Give the chemical reaction when A is treated with NaOH and name the reaction.
Answer the following:
What do you observe when glucose solution is heated with Tollen's reagent?
Name the monomers and the type of polymerisation in each of the following polymers:
1) Terylene
2) Polyvinyl chloride
Give balanced equation for the following reactions:
i) Ethylamine with nitrous acid.
ii) Diethyl ether with phosphorous pentachloride.
iii) Aniline with acetyl chloride.
Give balanced equations for the following name reactions:
i) Benzoin condensation
ii) Wurtz-Fitting reaction
iii) Carbylamine reaction
Give chemical test to distinguish:
i) Formaldehyde and acetaldehyde
ii) Dimethyl ether and alcohol.
