 Multiple Choice Questions
Multiple Choice QuestionsIn Fe(CO)5, the Fe – C bond possesses
π-character only
both σ and π characters
ionic character
ionic character
B.
both σ and π characters
The increasing order of the first ionization enthalpies of the elements B, P, S and F (lowest first) is
F < S < P < B
P < S < B < F
B < P < S < F
B < P < S < F
An ideal gas is allowed to expand both reversibly and irreversibly in an isolated system. If Ti is the initial temperature and Tf is the final temperature, which of the following statements is correct?
(Tf)irrev > (Tf)rev
Tf > Ti for reversible process but Tf = Ti for irreversible process
(Tf)rev = (Tf)irrev
(Tf)rev = (Tf)irrev
The decreasing values of bond angles from NH3 (106o ) to SbH3 (101o ) down group-15 of the periodic table is due to
increasing bp-bp repulsion
increasing p-orbital character in sp3
decreasing lp-bp repulsion
decreasing lp-bp repulsion
The “spin-only” magnetic moment [in units of Bohr magneton,(µB )] of Ni2+ in aqueous solution would be (Atomic number of Ni = 28)
2.84
4.90
1
1
The equilibrium constant for the reaction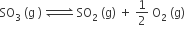
is Kc = 4.9 × 10–2. The value of Kc for the reaction'
2SO2 (g) +O2(g) ⇌ 2SO3 (g) will be
416
2.40 × 10–3
9.8 × 10–2
9.8 × 10–2
Following statements regarding the periodic trends of chemical reactivity of the alkali metals and the halogens are given. Which of these statements gives the correct picture?
The reactivity decreases in the alkali metals but increases in the halogens with increase in atomic number down the group
In both the alkali metals and the halogens the chemical reactivity decreases with increase in atomic number down the group
Chemical reactivity increases with increase in atomic number down the group in both the alkali metals and halogens
Chemical reactivity increases with increase in atomic number down the group in both the alkali metals and halogens
The enthalpy changes for the following processes are listed below:
Cl2(g) = 2Cl(g), 242.3 kJ mol–1
I2(g) = 2I(g), 151.0 kJ mol–1
ICl(g) = I(g) + Cl(g), 211.3 kJ mol–1
I2(s) = I2(g), 62.76 kJ mol–1
Given that the standard states for iodine and chlorine are I2(s) and Cl2(g), the standard enthalpy of formation for ICl(g) is
–14.6 kJ mol–1
–16.8 kJ mol–1
+16.8 kJ mol–1
+16.8 kJ mol–1
(∆H −∆U) for the formation of carbon monoxide (CO) from its elements at 298 K is
(R = 8.314 J K–1 mol–1)
–1238.78 J mol–1
1238.78 J mol–1
–2477.57 J mol–1
–2477.57 J mol–1
Which of the following chemical reactions depicts the oxidizing behaviour of H2SO4?
2HI+ H2SO4 →I2 +SO2+2HO
Ca(OH)2 +H2SO4 → CaSO4 +2H2O
NaCl +H2SO4 → NaHSO4 + HCl
NaCl +H2SO4 → NaHSO4 + HCl
