 Multiple Choice Questions
Multiple Choice QuestionsThe alkene that exhibits geometrical isomerism is
propene
2-methyl propene
2-butene
2-butene
C.
2-butene
2-butene may exist as 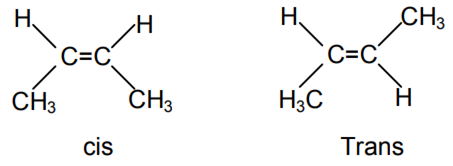
Due to restricted rotation around double bond, it exhibits geometric isomerism.
In which of the following arrangements, the sequence is not strictly according to the property written against it?
CO2< SiO2< SnO2< PbO2: increasing oxidising power
HF< HCl < HBr < HI : increasing acid strength
NH3< PH3< AsH3< SbH3: increasing basic strength
NH3< PH3< AsH3< SbH3: increasing basic strength
Which of the following statements is incorrect regarding physissorptions?
It occurs because of vander Waal’s forces.
More easily liquefiable gases are adsorbed readily.
Under high pressure, it results into multimolecular layer on the adsorbent surface.
Under high pressure, it results into multimolecular layer on the adsorbent surface.
Two liquids X and Y form an ideal solution. At 300 K, the vapour pressure of the solution containing 1 mol of X and 3 mol of Y is 550mm Hg. At the same temperature, if 1 mol of Y is further added to this solution, the vapour pressure of the solution increases by 10 mm Hg. Vapour pressure (in mmHg) of X and Y in their pure states will be respectively
200 and 300
300 and 400
400 and 600
400 and 600
The half life period of a first order chemical reaction is 6.93 minutes. The time required for the completion of 99% of the chemical reaction will be (log 2=0.301)
230.3 minutes
23.03 minutes
46.06 minutes
46.06 minutes
Given:  The value of standard electrode potential for the change
The value of standard electrode potential for the change  will be
will be
-0.072
0.385 V
0.770 V
0.770 V
On the basis of the following thermochemical data: (∆f G° H(aq)+=0)
H2O(l) → H+(aq) + OH–(aq); ∆H =57.32kJ
H2(g) + 1/2O2(g) → H2O(l); ∆H = –286.20 kJ
The value of enthalpy of formation of OH–ion at 25°C is
–22.88 kJ
–228.88 kJ
+228.88 kJ
+228.88 kJ
Copper crystallizes in fcc with a unit cell length of 361 pm. What is the radius of copper atom?
108 pm
127 pm
157 pm
157 pm
Which of the following has an optical isomer ?
[CO(en)(NH3)2]2+
[CO(H2O)(en)]3+
[CO(H2O)(en)]3+
Solid Ba (NO3)2 is gradually dissolved in a 1.0x 10−4 × Na2CO3 solution. At what concentration of Ba2+ will a precipitate begin to form?(Ksp for BaCO3 = 5.1 ×10−9 )
4.1 x 10-5 M
5.1 x 10-5 M
8.1 x 10-8 M
8.1 x 10-8 M
