 Multiple Choice Questions
Multiple Choice QuestionsAt 25°C, the solubility product of Mg(OH)2 is 1.0 × 10–11. At which pH, will Mg2+ ions start precipitating in the form of Mg(OH)2 from a solution of 0.001 M Mg2+ ions?
9
10
11
11
B.
10
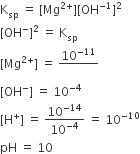
For a particular reversible reaction at temperature T, ΔH and ΔS were found to be both +ve. If Te is the temperature at equilibrium, the reaction would be spontaneous when
Te>T
T >Te
Te is 5 times T
Te is 5 times T
The time for half life period of a certain reaction A → Products is 1 hour. When the initial concentration of the reactant ‘A’ is 2.0 mol L–1, how much time does it take for its concentration to come from 0.50 to 0.25 mol L–1 if it is a zero order reaction?
1 h
4 h
0.25 h
0.25 h
A solution containing 2.675 of CoCl3. 6NH3(molar mass = 267.5 g mol–1) is passed through a cation exchanger. The chloride ions obtained is solution was treated with excess of AgNO3 to give 4.78 g of AgCl (molar mass = 143.5 g mol–1 ). The formula of the complex is
(At. mass of Ag = 108 u)
[Co(NH3)6]Cl3
[CoCl2(NH3)4]Cl
[CoCl3(NH3)3]
[CoCl3(NH3)3]
If sodium sulphate is considered to be completely dissociated into cations and anions in aqueous solution, the change in freezing point of water (ΔTf), when 0.01 mol of sodium sulphate is dissolved in 1 kg of water, is (Kf = 1.86 K kg mol–1).
0.0372 K
0.0558 K
0.0744 K
0.0744 K
29.5 mg of an organic compound containing nitrogen was digested according to Kjeldahl’s method and the evolved ammonia was absorbed in 20 mL of 0.1 M HCl solution. The excess of the acid required 15 mL of 0.1 M NaOH solution for complete neutralization. The percentage of nitrogen in the compound is
59.0
47.4
23.7
23.7
Which one of the following has an optical isomer?
[Zn(en)(NH3)2]2+
[Co(H2O)4(en)]3+
[Co(en)3]3+
[Co(en)3]3+
On mixing, heptane and octane form an ideal solution. At 373 K, the vapour pressures of the two liquid components (heptane and octane) are 105 kPa and 45 kPa respectively.Vapour pressure of the solution obtained by mixing 25.0 g of heptane and 35 g of octane will be (molar mass of heptane = 100 g mol–1 and of octane =114 g mol–1)
144.5 kPa
72.0 kPa
36.1 kPa
36.1 kPa
The edge length of a face centred cubic cell of an ionic substance is 508 pm. If the radius of the cation is 110 pm, the radius of the anion is
144 pm
288 pm
398 pm
398 pm
The Gibbs energy for the decomposition of Al2O3 at 500°C is as follows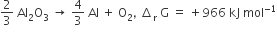
The potential difference needed for electrolytic reduction of Al2O3 at 500°C is at least
4.5 V
3.0 V
2.5 V
2.5 V
