 Multiple Choice Questions
Multiple Choice QuestionsWhich pair of the following carbonyl compounds can be diifferentiated by I2/NaOH?
C6H5-CHO and C6H5-CO-CH2-CH3
C6H5-CO-CH3 and CH3-CH2-CO-CH3
CH3-CH2-CO-CH2-CH3 and C6H5COCH2CH3
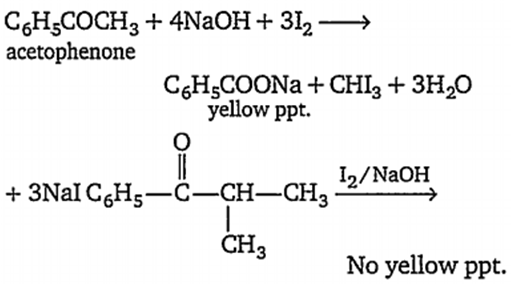
C6H5-CO-CH(CH3)-CH3 and C6H5-CO-CH3
D.
C6H5-CO-CH(CH3)-CH3 and C6H5-CO-CH3
Compounds containing -CO-CH3 (methyl carbonyl species) give a positive iodoform test with I2/ NaOH.
In the reaction sequence,
CH3CN . The products [A] and [B] are
![]()
(CH3)3COH, (CH3)2C=CH2
(CH3)2CHOH, CH3CH=CH2
CH3COCH3, CH3CH=CH2
In the carbylamine reaction, R-X is converted to R-Y via the intermediate Z. R-X, R-Y and Z, respectively, are
RNH2, RNC, Carbene
RNH2, RNC, nitrene
RNC, RNH2, carbene
ROH, RNC, nitrene
Which of the following statements is true?
Denaturation of protein changes the primary structure of protein
All proteins act as biocatalyst
C-terminal amino acid in proteins is determined by Edman degradation
The pleated sheet structure of proteins was determined by Pauling
Which one of the following is the strongest base ?
2,4,6-trinitroaniline
2,4,6-trinitro-N,N-dimethyl aniline
N, N-dimethyl aniline
Anline
The given reaction is called as
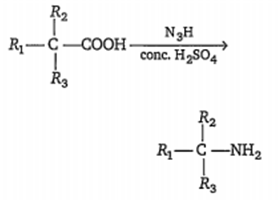
Schmidt rearrangement
Curtius rearrangement
Hofmann rearrangement
Lossen rearrangement
The boiling points of three isomeric pentanes 1, 2 and 3 are
(1) 9.5°C
(2) 28°C
(3) 36°C
1, 2 and 3 are respectively
n-pentane, iso-pentane, neo-pentane
iso-pentane, neo-pentane, n-pentane
n-pentane, neo-pentane, iso-pentane
neo-pentane, iso-pentane, n-pentane
