 Multiple Choice Questions
Multiple Choice QuestionsThe normality of 0.2M H3PO2 is
0.2 N
0.4 N
0.6 N
0.06 N
A.
0.2 N
The structure of H3PO2 is as
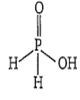
only ionisable H.
Therefore, it is a monobasic acid, and for monobasic acids M = N
0.2 M H3PO2 = 0.2 N H3PO2
NO is detected by ring test. Ring formed has formula
FeSO4.HNO2
FeSO4.NO2
[Fe(H2O)5.NO]2+
Fe(OH)2.NO
The process of oxidation involve
loss of electron
gain of electron
loss of proton
loss of neutron
The IUPAC name of the following compound
CH3 - CH = CH - C ≡ CH is
pent-2-en-4-yne
pent-1-en-4-yne
pent-3-en-1-yne
pent-2-en-5-yne
The number of electrons transferred when KMnO4 acts as an oxidising agent to give MnO2 and Mn2+ respectively are
2, 3
1, 5
3, 5
1, 3
