 Multiple Choice Questions
Multiple Choice QuestionsThe correct order of decreasing length of the bond as indicated by the arrow in the following structures is
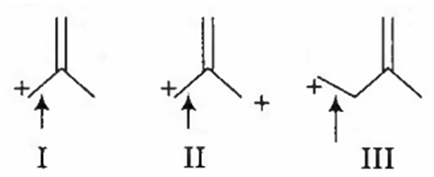
I >II > III
II > I > III
III > II > I
I > III > II
C.
III > II > I
The length of carbon-carbon single bond is always greater than that of the carbon-carbon double bond.
In III, positive charge is not in conjugation of double bond, so the bond does not acquire a double bond character. However, chances of acquiring double bond character (of the indicated bond) is much more in I than that in case of II.
Thus, the correct order of decreasing bond lenght is
An atomic nucleus having low n/p ratio tries to find stability by
the emission of an -particle
the emission of a positron
capturing an orbital electron (K-electron capture)
emission of a -particle
are example of
isotopes and isobars
isobars and isotones
isotones and isotopes
isobars and isotopes
98Cf246 was formed along with a neutron when an unknown radioactive substance was bombarded with 6C12. The unknown substance was
91Pa234
90Th234
92U235
92U238
The values of H and S of a certain reaction are -400 kJ mol-1 and -20 kJ mol-1K-1 respectively. The temperature below which the reaction is spontaneous, is
100 K
20C
20 K
120C
The correct statement regarding the following energy diagrams is
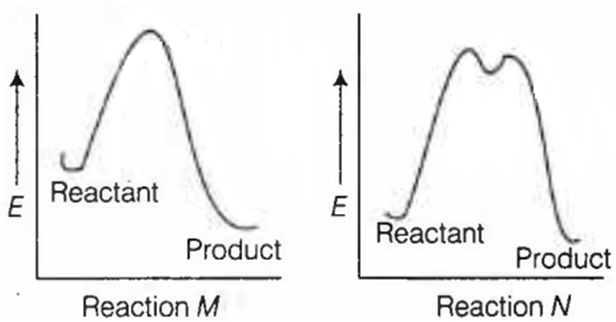
Reaction M is faster and less exothermic than reaction N
Reaction M is slower and less exothermic than reaction N
Reaction M is faster and more exothermic than reaction N
Reaction M is slower and more exothermic than reaction N
The enthalpy of vaporisation of a certain liquid at its boiling point of 35°C is 24.64 kJ mol-1. The value of change in entropy for the process is
704 JK -1mol-1
80 JK -1mol-1
24.64 JK -1mol-1
7.04 JK-1mol-1
In case of heteronuclear diatomics of the type AB, where A is more electronegative than B, bonding molecular orbital resembles the character of A more than that of B. The statement
is false
is true
cannot be evaluated since data is not sufficient
is true only for certain systems
